
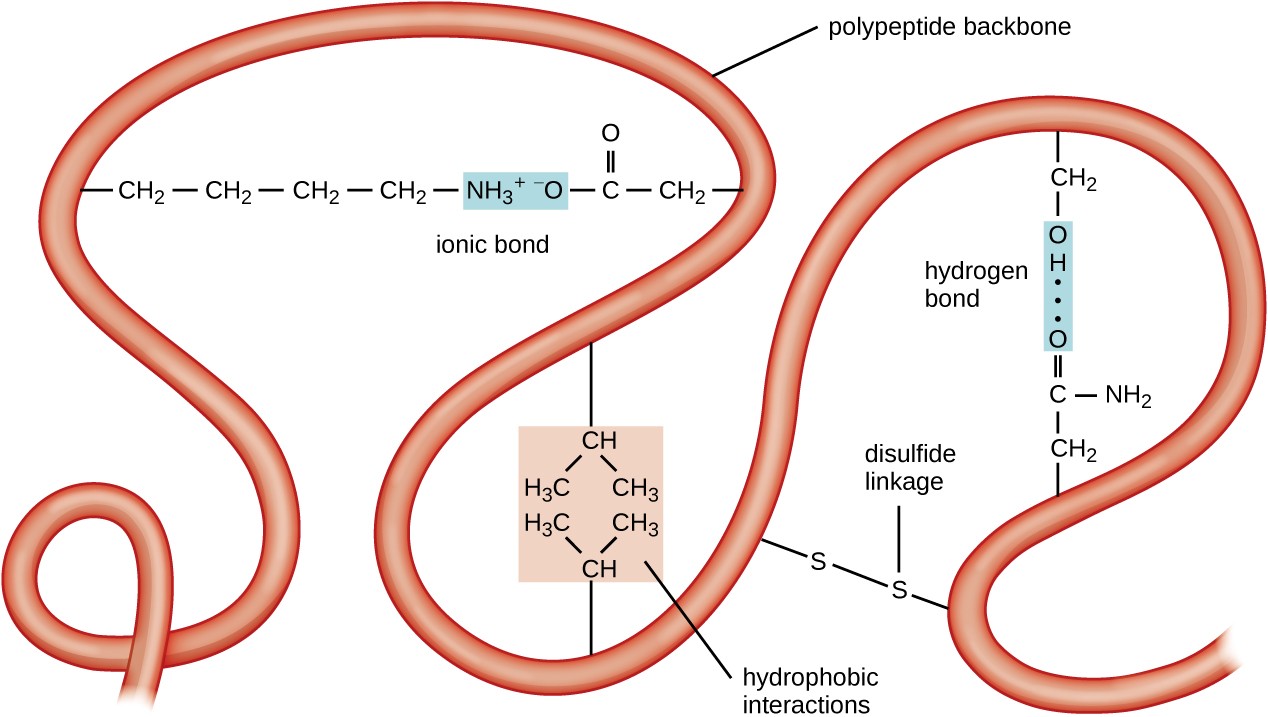
This is exemplified in sucrose (table sugar) which contains a linkage that is alpha to glucose and beta to fructose. In a traditional " chair structure" projection, if the linkage is on the same plane (equatorial or axial) as carbon 6 it is designated as beta and on the opposite plane it is designated as alpha. In a Fischer Projection, if the glycosidic linkage is on the same side or face as carbon 6 of a common biological saccharide, the carbohydrate is designated as beta and if the linkage is on the opposite side it is designated as alpha. The glycosidic linkages are designated as alpha or beta depending on the relative stereochemistry of the anomeric (or most oxidized) carbon. These backbone chains can be unbranched (containing one linear chain) or branched (containing multiple chains). This bond is called the glycosidic linkage. The backbone chain is characterized by an ether bond between individual monosaccharides. The polymers can be classified into oligosaccharides (up to 10 residues) and polysaccharides (up to about 50,000 residues). Carbohydrates Ĭarbohydrates arise by condensation of monosaccharides such as glucose. The synthesis of glycogen in the body is driven by the enzyme glycogen synthase which uses a uridine diphosphate (UDP) leaving group. A simplified example of condensation showing the alpha and beta classification. Spatial positions of backbone atoms can be reconstructed from the positions of alpha carbons using computational tools for the backbone reconstruction. For this reason, the primary structure of the amino acids in the polypeptide backbone is the map of the final structure of a protein, and it therefore indicates its biological function. Further interactions between residues of the individual amino acids form the protein's tertiary structure. Like almost all polymers, protein fold and twist, forming into the secondary structure, which is rigidified by hydrogen bonding between the carbonyl oxygens and amide hydrogens in the backbone, i.e. The sequence of the amino acids in the polypeptide backbone is known as the primary structure of the protein. Proteins are characterized by amide linkages (-N(H)-C(O)-) formed by the condensation of amino acids. Major families of biopolymers are polysaccharides (carbohydrates), peptides, and polynucleotides. Some uncommon but illustrative inorganic polymers include polythiazyl ((SN)x) with alternating S and N atoms, and polyphosphates ((PO 3 −) n). The silicon atoms bear two substituents, usually methyl as in the case of polydimethylsiloxane. Their backbond is composed of alternating silicon and oxygen atoms, i.e. Siloxanes are a premier example of an inorganic polymer, even though they have extensive organic substituents. Inorganic polymers Polydimethylsiloxane is classified as an " inorganic polymer", because the backbone lacks carbon. Major commercial products are polyethyleneterephthalate ("PET"), ((C 6H 4CO 2C 2H 4OC(O)) n) and nylon-6 ((NH(CH 2) 5C(O)) n). They have respectively -C(O)-O- and -C(O)-NH- groups in their backbones in addition to chains of carbon. Other major classes of organic polymers are polyesters and polyamides. Examples include polyolefins such as polyethylene ((CH 2CH 2) n) and many substituted derivative ((CH 2CH(R)) n) such as polystyrene (R = C 6H 5), polypropylene (R = CH 3), and acrylates (R = CO 2R'). Organic polymers Formation of polystyrene, a polymer with an organic backbone.Ĭommon synthetic polymers have main chains composed of carbon, i.e. Crystallization in its turn affects the optical properties of the polymers, its optical band gap and electronic levels.
polythiophenes) in thin films and in solution. The polymers with rigid backbones are prone to crystallization (e.g.
For example, in polysiloxanes (silicone), the backbone chain is very flexible, which results in a very low glass transition temperature of −123 ☌ (−189 ☏ 150 K).

its flexibility, determines the properties of the polymer (such as the glass transition temperature). Polymers are often classified according to the elements in the main chains. In polymer science, the polymer chain or simply backbone of a polymer is the main chain of a polymer. Selected which leads to the simplest representation of the That linear chain to which all other chains, long or short or both,Ĭould equally be considered to be the main chain, that one is


 0 kommentar(er)
0 kommentar(er)
